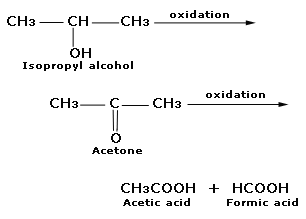
An alcohol A, when heated with concentrated H2SO4 gives an alkene B. When B is bubbled through bromine water and the product obtained is dehydrohalogenated with excess of sodamide, a new compound
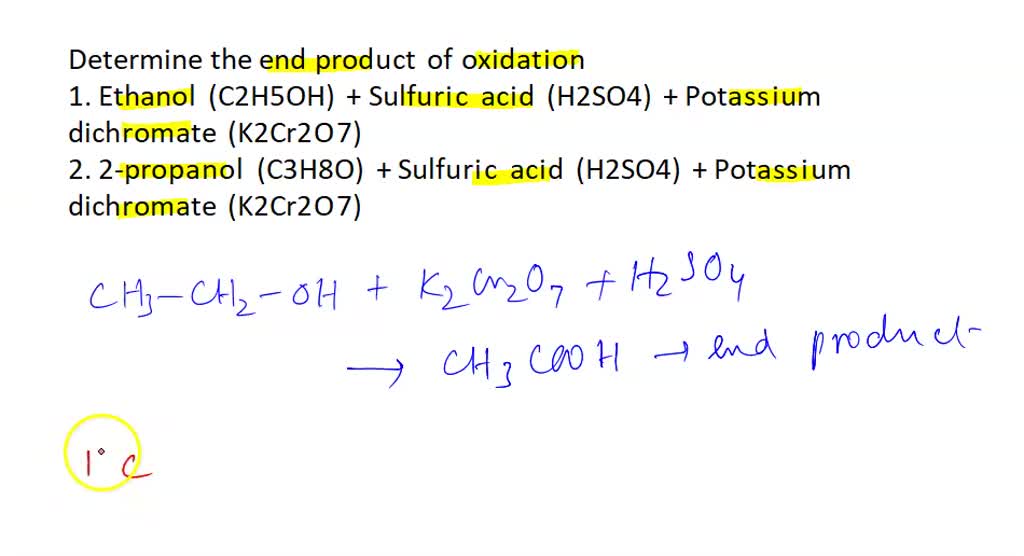
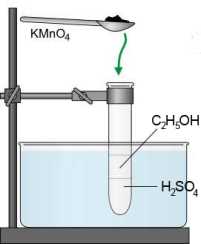
SOLVED: Oxidation of Alcohols Lab Questions Chemicals used Potassium Permanganate,KMnO4(aq) Water, H2O Concentrated Sulfuric Acid, H2SO4(aq) Ethanol Butan-1-ol Butan-2-ol 2-methylpropan-2-ol 1. What is the purpose of adding concentrated Sulfuric Acid in an
Carboxylic Acids and Derivatives – Synthesis Problems -Answers Please keep in mind that there may be multiple options for answ